Atomic Structure
Atom
The smallest particle of an element is called an atom. An atom can take part in chemical combination and does not occur free in nature. The atom of the hydrogen is the smallest and lightest.
Example — Na, K, Ca, H etc.
Molecule
A molecule is the smallest particle of an element or compound that can have a stable and independent existence.
Example — O2, N2 Cl2, P4 , S8 etc.
Mole
A mole is a collection of 6.023 x 1023 particles. It means that
1 mole = 6.023 x 1023
1 mole atom = 6.023 x 1023 atoms
1 mole molecule = 6.023 x 1023 molecules
1 mole ion = 6.023 x 1023 ions
Avogadro's Number :
The number 6.023 x 1023 is called Avogadro's Number.
Atomic Mass
It is the ratio of mass of one atom of the element to 1/12th part of the mass of one atom of carbon-12.
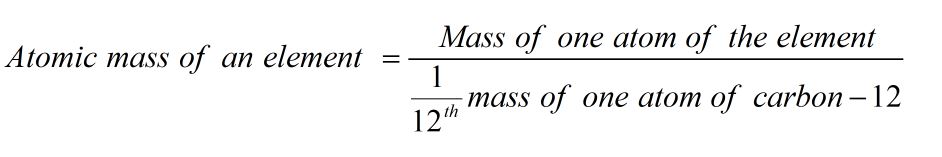
Actual mass of 1 atom of an element = atomic mass in amu x 1.66 x 10-24 g
Molecular Mass
It indicates how many times one molecule of a substance is heavier in comparison to 1/12th mass of one atom of Carbon-12.
