Diffusion of gas
Diffusion of gas
The process of intermixing of gases irrespective of the density relationship and without the effect of external agency is called diffusion of gases.
In a gas, the molecules are far separated and the empty space among the molecules are very large. Therefore the molecules of one gas can move into the empty spaces or voids of the other gas and vice-versa. This leads to diffusion.
Graham's law of diffusion
Under the similar conditions of temperature and pressure, the rates of diffusion of gases are inversely proportional to the square roots of their densities.
Let r1 and r2 be the rates of diffusion of two gases A and B, d1 and d2 be their respective densities, then according to Graham's law of diffusion.
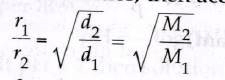
Since molecular mass = 2 × vapour density.
M = 2 × d
Dalton's law of partial pressure
It states that- If two or more gases which do not react chemically are enclosed in a vessel, the total pressure of the gaseous mixture is equal to the sum of the partial pressure that each gases which exert pressure when enclosed separately in the same vessel at constant temperature.
Let p1, p2 and p3 be the pressure of three non-reactive gases when enclosed separately. Let total pressure be p then
p = p1 + p2 + p3
