Matter in our surrounding
Matter in our surrounding
Matter is anything which has mass and occupies space. All solids, liquids, and gases around us are made of matter. Scientists believe that matter is made of tiny particles that clump together. A substance is a pure kind of matter having only one kind of constituent particle (atom or molecule). Water, iron, gold, copper, aluminium, and oxygen are examples of substances. All substances are matter, but all forms of matter are not substances.
Matter can ordinarily exist in three states—solid, liquid, and gas. These three states of matter have different properties. Water exists in all the three states namely steam or water vapour (gas), water at room temperature (liquid), and ice (solid). This is the only substance that exists naturally in all the three states.
Matter can be classified in many ways. However, the following are the two main ways of classifying the matter :
1. By the physical state of matter as a solid, liquid, or gas.
2. By the chemical composition of matter as an element, a compound, or a mixture.
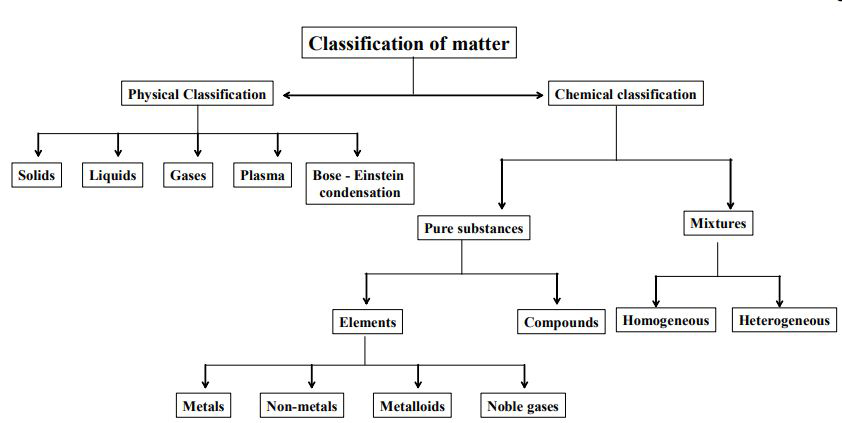
Classification of matter based on physical states
Matter can ordinarily exist in three states —
1. Solid
2. Liquid
3. Gas
Water exists in all the three states namely steam or water vapour (gas), water at room temperature (liquid) and ice (solid). This is the only substance that exists naturally in all the three states.
❖ The characteristic properties of different states of matter depend on intermolecular forces. The forces holding molecules together are called intermolecular forces. Intermolecular forces try to keep molecules together, but thermal energy always tries to keep them far apart.
❖ It is the competition between molecular interaction energy and thermal energy that decides whether a given substance under given conditions will be a solid, liquid, or gas.
❖ Thermal or heat energy can convert one state of matter into another state. Thus, a particular state of matter depends on both intermolecular force and the thermal energy that basically depends upon temperature.
