Acid Base & Salts
Acid Base & Salts
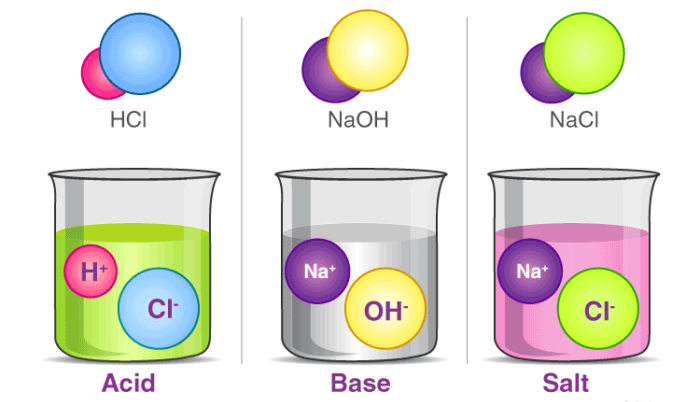
Acid
The term acid is derived from a Latin word ‘acidus’ or ‘acere’, which means sour. The most common characteristic is their sour taste.It turns blue litmus paper red. These dissociate in their aqueous solution to form their constituent ions, as given by the following examples.
Based on their occurrence, they are divided into two types - Natural and mineral acids
Natural Acids :
These are obtained from natural sources, such as fruits and animal products.
For e.g. lactic, citric, and tartaric acid etc.
Mineral Acids :
Mineral acids are acids prepared from minerals.
Examples are Hydrochloric acid (HCl), Sulphuric Acid (H2SO4), and nitric acid (HNO3), etc.
Base
The most common characteristic of bases is their bitter taste and soapy feel. A base is a substance that renders hydroxyl ion(OH–) in their aqueous solution. Bases turn the colour of red litmus paper to blue.
Examples : Sodium hydroxide (caustic soda) – NaOH, Potassium hydroxide (caustic potash) – (KOH)
Bases can be divided in two types – Water soluble and Water-insoluble
Salt
Salt is an ionic compound that results from the neutralization reaction of acids and bases. Salts are constituted of positively charged ions, known as cations, and negatively charged ions, known as anions, which can either be organic or inorganic in nature. These ions are present in a relative amount, thus rendering the nature of the salt neutral.