Model of an Atom
Model of an Atom
According to Thomson, an atom is treated as sphere of radius 10-8 cm in which positively charged particles are uninform ally distributed and negatively charged electrons and embedded through them. This is also called Plum-Pudding model of an atom or watermelon model of an atom.
Rutherford's model of an atom
On the basis of scattering experiment, Rutherford proposed a model of the atom which is known as nuclear atomic model.
According to this model :
(i) An atom consists of a heavy positively charged nucleus where all protons and neutrons are present. Protons & neutrons are collectively called nucleons. Almost whole mass of the atom is contributed by these nucleons.
(ii) Radius of a nucleus = 10-13 cm
Radius of an atom = 10-8 cm
Radius of an atom = 105 times of the radius of the nucleons.
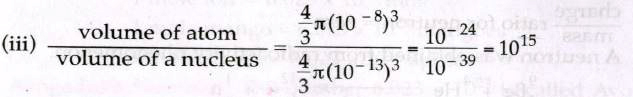
So, volume of an atom is 1015 times heavier than volume of a nucleus.
(iv) Electrons revolve around the nucleus in closed orbits with high speed. This model is similar to the solar system, the nucleus representing the sun and revolving electrons as planets. The electrons are therefore, generally referred as planetary electrons.
| Thomson's model | Plum pudding model (watermelon model) |
| Rutherford's model | Nuclear theory |
| Bohr's model | Concept of Quantization of energy. |
| Planck's Quantum theory | Photon & quanta |
| Sommerfeld's model | Orbital : elliptical & spherical |
| de-Broglie's equation | Dual nature of electron |
| Heisenberg's Uncertainty principle | Exact position & momentum can not be determined simultaneously |
| Schrodinger's wave equation | wave nature of electron. |
