Chemical Bonding
Chemical Bonding
Atoms are held together in compounds by the forces of attraction which result in formation of chemical bonds. The formation of chemical bonds results in the lowering of energy, which is less than the energy of the individual atoms. The resulting compound is lower in energy when compared with sum of energies of the reacting atom/molecule and, hence, is more stable.
There are many types of chemical bond :
» Ionic bond or (Electrovalent bond)
» Covalent bond
» Co-ordinate bond (or Dative bond)
» Sigma bond (σ-bond)
» Pi-bond (π-bond)
Ionic bond or (Electrovalent bond)
A bond formed by the complete transfer of one or more electrons from one atom to other atom is called ionic bond.
Example : when sodium metal and chlorine gas are brought into contact, they react violently and we obtain sodium chloride. This reaction is shown below:
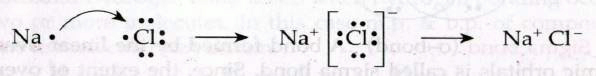
Condition of ionic bond
I. Ionization energy of metal should be low.
II. Electron affinity of non-metal should be high.
Properties of ionic compounds
(a) Ionic compounds have high melting point & boiling point.
(b) Ionic compounds are crystalline solids. In the crystal, the ions are arranged in a regular fashion. The ionic compounds are hard and brittle in nature.
(c) Ionic compounds are good conductor of electricity in molten state or in water.
(d) Ionic compounds are bad conductor of electricity in solid state.
(e) Ionic compounds are soluble in water.
(f) Ionic compounds are insoluble in non-polar covalent like Benzene, Carbon tetrachloride etc.
Covalent bond
A bond formed between two same or different atoms by mutual contribution and sharing of electrons is called covalent bond. It is this lowering of energy that leads to the formation of the covalent bond.
Example :

Properties of covalent compounds :
(a) The covalent compounds consist of molecules that are electrically neutral in nature. The forces of attraction present between the molecules are less strong when compared with the forces present in ionic compounds.
(b) Because of the weak forces of attraction present between discrete molecules, called intermolecular forces, the covalent compounds exist as a gas or a liquid or a solid.
(c) The melting points and boiling points of covalent compounds are lower than those of ionic compounds.
(d) The covalent compounds contain neutral molecules and do not have charged species such as ions or electrons which can carry charge.
(e) Covalent compounds are generally not soluble in water but are soluble in organic solvents such as alcohol, chloroform, benzene, ether, etc.
Co-ordinate bond (or Dative bond)
Co-ordinate bond is a special type of covalent bond in which one atom donates electrons to other atom. The bonding between donor to acceptor atom is called co-ordinate bond.
Example :
Sigma bond (σ-bond)
A bond formed by the linear overlapping of atomic orbitals is called sigma bond. Since, the extent of overlapping of atomic orbitals in a-bond in large. Hence a-bond is a strong bond.
Pi-bond (π-bond)
A bond formed by the sidewise (or lateral overlapping of atomic orbitals is called pi-bond, since, in this case, extent of overlapping of atomic orbitals is lesser than a-bond. So, re-bond is a weak bond.

Bond energy
The amount of energy required to break one mole bonds of a particular type between the atoms in the gaseous state of a substance is called bond energy. The bond energy depends upon the following factors —
I. Size of atom
II. Multiplicity of bonds.
» Greater the size of atoms, Lesser will be bond energy.
» Greater the bond multiplicity more will be bond energy.
Bond energy : Single bond < double bond < triple bond
Bond Length
The average equilibrium distance between the centers of the two bonded atoms is called bond length. The bond length is influenced by the following factors —
I. Size of atoms
II. Multiplicity of bonds
» Greater the size of atoms, greater will be bond length.
» Greater the multiplicity of bonds, lesser will be bond length.
