Constituents of an Atom
Constituents of an Atom
Fundamental particles of an atom are Electron, Proton & Neutron.
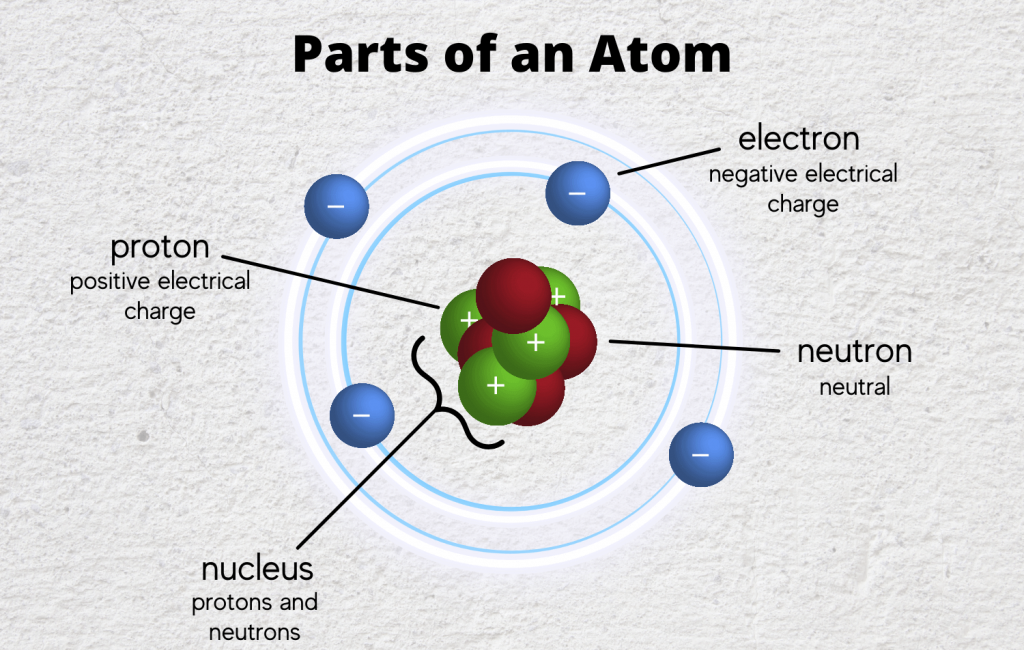
Electron
(i) Electron had been discovered by J.J. Thomson.
(ii) The name of electron was given by Stoney.
(iii) Charge on an electron
Relative : -1 unit
Absolute : -1.6 x 10-19 coulomb or -4.8 x 10-10 e.s.u.
(iv) Mass of an electron
Relative : 0.000543 amu
Absolute : 9.1 x 10-28 g
(v) Charge / mass (e/m) ration of electron = -1.76 x 108 C/g
(vi) An electron was obtained from cathode rays experimental.
Proton
(i) A proton had been discovered by Goldstein.
(ii) A proton was named by Rutherford.
(iii) Charge on proton
Relative : +1 unit
Absolute : +1.6 x 10-19 coulomb or +4.8 x 10-10 e.s.u.
(iv) Mass of an proton
Relative : 0.000763 amu
Absolute : 1.67 x 10-24 g
(v) Charge / mass (e/m) ration of electron = 9.58 x 104 C/g
(vi) An proton was obtained from anode rays experiment.
Neutron
(i) A neutron had been discovered by James Chadwick.
(ii) Charge on neutron - zero
(iii) Mass of an neutron
Relative : 1.000863 amu
Absolute : 1.675 x 10-24 g
(iv) Charge / mass (e/m) ration of electron = 0
(v) A neutron was obtained from radioactivity phenomenon.
